ORIGINAL RESEARCH
Formulation and Characterization of Gels Containing Natural and Modified Bromelain
Liana Nadirashvili,1 Tamari Korinteli,2 Nana Gorgaslidze,2,ID Malkhaz Getia,3,ID Aliosha Bakuridze4,ID
Received: 28 November 2023; Accepted: 23 December 2023; Available online: 29 February 2024
ABSTRACT
Background: Bromelain is a complex of plant-derived proteolytic enzymes found in almost all parts of the Pineapple plant (Ananas comosus L.Merr.) and is currently used in treating muscle-skeletal disorders.
Objectives: This study aimed to develop natural and modified Bromelain gels for potentially treating muscle-skeletal disorders and assessing their physical-chemical properties. Natural Bromelain and its chemically altered form, modified with dextran aldehyde, were used as active ingredients.
Methods: Investigation of physical and chemical properties of gels, identification of gels by Fourier transform infrared (FT-IR) spectroscopy.
Results: The proteolytic activity of the gels for 15 months is stable and remains 85-93% compared to the initial activity.
Conclusions: The spreadability, consistency, pH, thermal, and colloidal stability of the base and gels containing natural and modified Bromelain were satisfactory.
Keywords: Bromelain; Carbopol 940; dextran aldehyde; muscle-skeletal disorders.
DOI: 10.52340/GBMN.2023.01.01.59
INTRORDUCTION
Muscle-skeletal disorders (MSDs) are one of the primary reasons people seek medical care worldwide. They comprise more than 150 diagnoses that affect the locomotor system, as listed in the International Classification of Diseases.1,2
Non-steroidal anti-inflammatory drugs (NSAIDs) are the medications of choice for musculoskeletal disorder-associated acute pain management. However, they manifest several side effects as a significant number of the patients with MSDs reportedly experienced dizziness, abdominal pain, indigestion, and gastric ulcers from NSAIDs.3 Therefore, the search for new MSD remedies and the selection of the best dosage forms for them remains one of the significant challenges of modern medicine.
Plant enzymes are characterized by a wide range of therapeutic activity and are used in treating various diseases, including muscle-skeletal disorders.4 One such enzyme is Bromelain, a proteolytic enzyme complex found in almost all parts of the Pineapple plant (Ananas comosus L. Merr.).5 It is known the possible application of Bromelain in treating of cardiovascular diseases, blood coagulation and fibrinolysis disorders, infectious inflammation-associated diseases, and many types of cancer.6-8
Its wide range of therapeutic activities also includes strong anti-inflammatory and analgesic effects.9 Bromelain is an effective treatment for MSDs. Bromelain is used as a benign substitute for NSAIDs, and researchers have identified significant efficacy of Bromelain in arthritis.10 Proteolytic enzymes of plant origin sometimes cause allergic reactions, and their chemical modification is one of the most efficient ways of reducing such a side effect.11-14 The topical administration of drugs to achieve optimal cutaneous and percutaneous drug delivery has recently gained importance.
Among the other semisolid dosage forms, gels have many advantages: they are an elegant, non-greasy formulation, biodegradable, biocompatible, and easy to formulate; they provide excellent spreadability and cooling effect because of solvent evaporation; they have comparatively fewer long-term stability issues; they can be used to administer both polar and non-polar drugs.15
One of the most commonly used gelling agents is Carbopol 940, a non-toxic and non-irritable acrylic polymer that forms a transparent and bio-adhesive gel. It influences multiple properties of semisolids, such as pH, viscosity, spreadability, adhesion, organoleptic properties, and stability. In controlled-release drug formulations, Carbopol 940 is commonly used.16
In previous work, we prepared natural and modified Bromelain gels based on the polyethylene glycol-1500 and polyethylene glycol-4000 mixtures.17 After six months, the samples' proteolytic activities were reduced. The work aims to formulate a more stable natural and modified Bromelain gel with Carbopol 940 as the base.
METHODS
Study materials
Commercial Bromelain obtained from the stem of the Pineapple plant (Ananas comosus L. Merr.) was purchased from Beijing Wisapple Biotech Co., Ltd; dextran (molar mass 35 -40 kDa), sodium borohydride, potassium periodate, L-cysteine, KBr and Sephadex G-75 – from Sigma Aldrich; Casein – from Carl Roth; Carbopol 940, almond oil, nipagin, tween 80, potassium sorbate, propylene glycol - from Lubrizol.
Study equipment
Spectrophotometric analysis was conducted in quartz cuvettes (10 mm) on a Jasco V-730 UV-Vis spectrophotometer. The spectra were automatically processed by UV-Probe system software (version 2.14.02). pH was determined by a pH meter Milwaukee – MW 150 MAX; rheology was studied by a DahoMeter DH-DJ-8S viscometer; bioavailability was determined on the Vertical Franz Diffusion apparatus EMFDC-07; a light microscope ApoTome1 2nd floor inverse CRTD (Zeiss) was used for microscopy; colloidal stability was studied using a Centrifuge Metronex, 310. IR spectra were obtained using a Jasco FT/IR-4600 spectrometer.
Chemical modification of Bromelain
Natural Bromelain was chemically modified using dextran aldehyde and later purified by gel filtration on Sephadex G-75, as described in our previous article.18 Protein concentration in Bromelain was determined by the Extinction coefficient method.19
Preparation of the base and gels
The base was prepared by mixing the oil-based phase (almond oil, nipagin, tween 80) with the aqueous phase (Carbopol 940 with a concentration of 1.5%, potassium sorbate, propylene glycol) at room temperature under stirring until complete dispersion and the formation of a uniform mass. To develop the gels with natural Bromelain and modified Bromelain, they were introduced into the oil phases, and then the oil-based phases with enzymes were mixed with the aqueous phases.
Physical and chemical properties of the base, natural, and modified Bromelain gels
The quantitative analysis of the gels was done using the proteolytic activity determination method.20 Some features of the prepared gels, such as color and odor, were assessed organoleptically—the influence of storage time on the proteolytic activity. The proteolytic activity of the natural and modified Bromeline gels was studied at +5-7C0 during 15-month period.
Rheological Study/ Viscosity
The viscosity of base, natural, and modified Bromelain gels was studied using a viscometer. Samples were allowed to settle over 30 min at a temperature of 25 ±/1oC before the measurements were taken. Viscosity was reported in cP.
Spreadability
Spread ability was determined according to Knorst.21
pH measurement
pH was determined by a pH meter Milwaukee – MW 150 MAX.
Thermal stability
The glass test tubes (diameter of 15 mm and a height of 150 mm) were filled with the research objects (base, natural Bromelain gel, modified Bromelain gel) in the amount of 8-10 ml and placed in a thermostat (40°C) for one week to determine the thermal stability of the samples. Then, they were placed in a refrigerator (10-12ºC) for another week, and after that, they were kept at room temperature for three days and nights.22,23
Colloidal stability
Test tubes were filled with the samples (base, natural Bromelain gel, modified Bromelain gel) up to 2/3 of the volume, weighed with an accuracy of 0.01 g, placed in a centrifuge, and centrifuged for 5 minutes at 6000 rpm.24
Identification
Identification of the base, natural, and modified Bromelain gels was carried out using FT-IR spectroscopy.25
RESULTS AND DISCUSSION
The influence of storage time on the proteolytic activity of natural Bromelain and modified Bromelain gels is shown (Tab.1).
TABLE 1. Influence of storage time on the proteolytic activity of natural Bromelain and modified Bromelain gels

It was revealed that the proteolytic activity of the gels for 15 months is stable and remains 85-93% compared to the initial activity.
Organoleptic characteristics
All study samples were pleasant to the touch and easily applicable on the skin, homogeneous, shiny, with a weak characteristic odor. The base was white, the natural Bromelain gel was yellowish-white, and the modified Bromelain gel was slightly yellowish-white in color.
Rheological characteristics/Viscosity
The results show that the systems are characterized by non-Newtonian flow (Fig.1 and Fig.2). When the rotation speed increases, the viscosity decreases; when the speed decreases, it increases. The viscosity of the research objects varied between 572-15223 cP.
FIGURE 1. Dependence of viscosity (cp) of base (A), natural (B), and modified (C) Bromelain gels on spindle speed (rpm); (light green: ascending; dark green: descending)

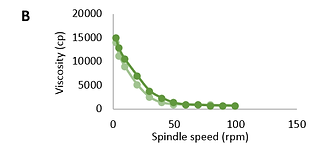
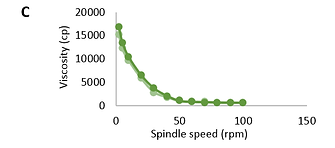
FIGURE 2. Dependence of deformation speed (s-1) of base (A), natural (B), and modified (C) Bromelain gels on share stress (mpa); (light green: ascending; dark green: descending)

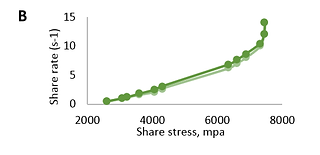
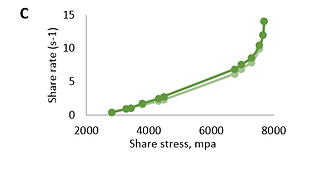
Based on the performed rheological studies, it can be concluded that the research samples represent dispersed systems with elastic-plastic properties. Flow curves on the rheogram show that the rheological properties of the studied gels practically do not differ. The ascending and descending curves of the hysteresis loop (which overlap each other) indicate that the studied samples have thixotropic properties.
Determination of spreadability
As the calculations showed, the base spreadability and spreadability factor were higher than that of the modified Bromelain; however, the difference was insignificant (Fig.3 and Fig.4).
FIGURE 3. Determination of spreadability of base, natural, and modified Bromelain gels

FIGURE 4. Determination of spreadability factor of base, natural, and modified Bromelain gels

Determination of pH
The average pH values of the base, natural, and modified Bromelain were 5.63, 5.54, and 5.55, respectively. The pH of all the formulations indicates that they are acceptable to avoid the risk of irritation upon application to the skin.
The thermal stability tests showed that the base, natural, and modified Bromelain gels did not separate, and they maintained the initial color. Therefore, they have high thermal stability.
After the colloidal stability test, no segregation was observed visually in the base, natural, and modified Bromelain gels. So, they were considered to be colloidally stable.
Identification
Identification of the base, native, and modified Bromelain gels was carried out using Fourier transform infrared (FT-IR) spectroscopy (Fig.5).
FIGURE 5. FT-IR spectra (KBr) of the base (A), native Bromelain (B), and modified Bromelain (C)

The FT-IR spectrum (Fig.5A) of Carbopol 940 (C 940) was in agreement with the reported data (USP 29, 2006). In the case of C 940, the FT-IR spectra peaking at 2925.48 cm-1 represented OH stretching vibration, i.e., ν OH and intramolecular hydrogen bonding. The prominent band at 1744.3 cm-1 was assigned to carbonyl C=O stretching vibration, i.e., ν C=O. While the peak at ν 1454.06 cm-1 was for COO-H, the band at 1287.57 cm-1 was due to ν C-O-C of acrylates, the band at 802.242 cm-1 was for out-of-plane bending of C=CH., δ=C-H19,22.
On the spectrum (Fig.5B), the prominent characteristic peaks of Carbopol 940 were identified as -2925.48 cm-1, 1745.26 cm-1, 1456.96 cm-1, 1285.32 cm-1 and 801.278 cm-1. The following peaks of Bromelain were detected: N–H stretch bands at 3417.24 cm-1 and C–H stretches at 2925,48 cm−1, which overlaps the ν O-H bond of Carbopol. Strong intensities of C=O stretch bands (amide 1 area) at 1646.91 cm-1, C–N stretch bands at 1538.92 cm-1, aliphatic amine at 1250.61 cm-1, C–H bending of aromatic residue of tryptophan or tyrosine at 890.952, at 748 N–H wagging of secondary amide and 679.785 C–S stretch of sulfides and disulfides.
Both Carbopol- 940's (2930.31 cm-1, 1745.26 cm-1, 1456.96 cm-1, 1279.54 cm-1 and 804.17 cm-1) and Bromelain (3404.02 cm-1, 2930.31cm−1, 1646.91 cm-1, 1536.99 cm-1, 1250.61 cm-1, 752.382 cm-1, 679.785 cm-1) prominent characteristic peaks were identified (Fig.5C). Regarding dextran, the peaks at 3504.02 cm−1 and 3312.14 cm−1 are attributed to the O-H stretching; the band at 1645.95 cm−1 corresponds to water molecule bending. Furthermore, the peaks at 2930 (these peak overlaps with those of Carbopol and Bromelain) and 1419 cm−1 are assigned to the v (C‒H) and δ (C‒H) vibrational modes the peaks at 1161.9 illustrates C-O stretching vibrations, and the peaks at 916.022 cm−1 and 852.382 cm−1 demonstrates α-glucopyranose ring deformation mode.
FT-IR was used to recognize the occurrence of different functional groups present in the samples. The method allowed the identification of the base, native, and modified Bromelain. Also, due to the detection of dextran peaks, natural and modified Bromelain can be distinguished from each other. Therefore, the method can be used for the standardization of given gels.
CONCLUSIONS
As a result of the present study, formulations for natural and modified Bromelain gels were developed based on Carbopol 940. The developed base and gels comply with all quality parameters. The base and gels' spreadability, consistency, pH, thermal, and colloidal stability were satisfactory. Proteolytic activity was used for quantitative assessment, while FT-IR spectroscopy allowed the identification of the formulated base and gels. The modified Bromelain gel can be used as an efficient medication for treating muscle-skeletal disorders.
AUTHOR AFFILIATION
1 Department of Phytochemistry, Direction of Fermentology, I.Kutateladze Institute of Pharmacochemistry, Tbilisi State Medical University, Tbilisi, Georgia;
2 Department of Social and Clinical Pharmacy, Tbilisi State Medical University, Tbilisi, Georgia;
3 Department of Pharmaceutical Analysis and Standartisation, I. Kutateladze Institute of Pharmacochemistry of Tbilisi State Medical University, Tbilisi, Georgia;
4 Department of Pharmaceutical Technology, Tbilisi State Medical University, Tbilisi, Georgia.
REFERENCES
-
World Health Organization. Musculoskeletal health. 2022. Available at: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions
-
James, S. L., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018;392: 1789-858.
-
Onigbinde Ayodele Teslim, M’Kumbuzi Vyvienne, Olaogun Mathew Olatokunbo, Afolabi Joshua Oluwafisayo, Nondwe Bongokazi Mlenzana, Manie Shamila, Tarimo Nesto, Mukoka Grace. Side Effects of Non-Steroidal Anti-Inflammatory Drugs: The Experience of Patients with Musculoskeletal Disorders. American Journal of Health Research 2014;2(4):106-112
-
Silva-López RE, Gonçalves RN. Therapeutic proteases from plants: biopharmaceuticals with multiple applications. JABB 2019;28;6(2):101–9.
-
Rathnavelu V., Alitheen N., Sohila S., Kanagesan S and Ramesh R. Potential role of bromelain in clinical and therapeutic applications (Review). Biomedical reports 2016;5(3), 283–288.
-
P. Hikisz and J. Bernasinska-Slomczewska. Beneficial Properties of Bromelain. Nutrients. 2021 Dec; 13(12): 4313. doi: 10.3390/nu13124313.
-
R. Pezzani, M. Jiménez-Garcia, X. Capó, E. Sönmez Gürer, F. Sharopov, 6T. Yamthe Lauve Rachel, D. N. Woutouoba, A. Rescigno, S. Peddio, P. Zucca, P. Valere T. Fokou, M. Martorell, Z. Gulsunoglu-Konuskan, A. Ydyrys, T. Bekzat, G.Tussupbekova, C. Hano, J. Sharifi-Rad, and D. Calina . Anticancer properties of bromelain: State-of-the-art and recent trends. Front Oncol. 2022; 12: 1068778. Published online 2023 Jan 9. doi: 10.3389/fonc.2022.1068778.
-
Jung-Ha Lee, Jin-Tae Lee, Hae-Ryoun Park, Jin-Bom Kim. The potential use of bromelain as a natural oral medicine having anticarcinogenic activities. Food Sci Nutr. 2019 Apr 1;7(5):1656-1667. doi: 10.1002/fsn3.999. eCollection 2019 May.
-
Akhtar N., Naseer R., Farooqi A., Aziz W & Nazir M. Oral enzyme combination versus diclofenac in the treatment of osteoarthritis of the knee – a double-blind prospective randomized study. Clinical Rheumatology 2004;23(5), 410-5.
-
Chakraborty, A. J., Mitra, S., Tallei, T. E., Tareq, A. M., Nainu, F., Cicia, D., … Capasso, R. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life 2021;11(4), 317.
-
Bencharitiwong R., Kleij H., Koppelman S., Nowak-Węgrzyn A. Effect of chemical modifications on allergenic potency of peanut proteins. Allergy and asthma proceedings 2015;36(3), 185–191.
-
Nwagu TN, Ugwuodo CJ. Stabilizing bromelain for therapeutic applications by adsorption immobilization on spores of probiotic Bacillus. Int J Biol Macromol 2019;15;127:406–14.
-
Xue Y, Wu CY, Branford-White CJ, Ning X, Nie HL, Zhu LM. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity. Journal of Molecular Catalysis B, Enzymatic 2010;3–4(63):188–93.
-
Brito AMM, Oliveira V, Icimoto MY, Nantes-Cardoso IL. Collagenase Activity of Bromelain Immobilized at Gold Nanoparticle Interfaces for Therapeutic Applications. Pharmaceutics 2021;13(8):1143.
-
Labarre D, Ponchel G, Vauthier C. Biomedical and Pharmaceutical Polymers. Pharmaceutical Press, London, UK, 2010
-
Safitri F, Nawangsari D, Febrina D. Overview: Application of Carbopol 940 in Gel. Proceedings of the International Conference on Health and Medical Sciences (AHMS 2020) In 2021.
-
Korinteli T., Gorgaslidze N., Bakuridze A., Nadirashvili L., Erkomaishvili G. Composition and Technology of natural and modified bromelain gels. Experimental and Clinical Medicine, (2022), No. 6, pp. 8-13.
-
Korinteli T, Gorgaslidze N, Nadirashvili L, Erkomaishvili G. Chemical Modification of Bromelain with Dextran Aldehyde and Its Potential Medical Application. Georgian Med News. 2021 Jun;(315):185–189.
-
Gupta P., Saleemuddin M. B Oriented immobilization of stem bromelain via lone histidine on metal affinity support. Journal of Molecular Catalysis B Enzymatic, 2007, 45(3-4), 78-83.
-
Kaverzneva E.D. Standard method for determining proteolytic activity for complex protease preparations. Appl. Biochem. And microbiol. 1971, 7(2), 225-228.
-
M.T. Knorst, Desenvolvimento tecnológico de forma farmacêutica plástica contendo extrato concentrado de Achyrocline satureioides. (Lam). DC. Compositae. (Marcela), M. Sc. thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, 1991, p. 257.
-
Deuschle, V. C. K. N., Deuschle, R. A. N., Bortoluzzi, M. R., & Athayde, M. L. (2015). Physical chemistry evaluation of stability, spreadability, in vitro antioxidant, and photo-protective capacities of topical formulations containing Calendula officinalis L. leaf extract. Brazilian Journal of Pharmaceutical Sciences, 51(1), 63–75. doi:10.1590/s1984-82502015000100007
-
Butkeviciute, A.; Ramanauskiene, K.; Janulis, V. Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 373. https://doi.org/10.3390/ antiox11020373
-
Aciole I, Andrade Júnior FPD, Cordeiro L, Souza J. Aloe gel: manipulation and characterization of physical-chemical quality adjustment. Revista Colombiana de Ciencias Químico Farmacéuticas. 2020 Dec 1;49:790–805.
-
Sahoo S, Chakraborti CK, Naik S, Mishra SC, Nanda UN. Structural Analysis of Ciprofloxacin-Carbopol Polymeric Composites by X-Ray Diffraction and Fourier Transform Infra-Red Spectroscopy. Tropical Journal of Pharmaceutical Research [Internet]. 2011 [cited 2022 Jul 6];10(3). Available from: https://www.ajol.info/index.php/tjpr/article/view/67938.
